Salmonella species, Listeria monocytogenes, Shigella species and verotoxigenic E.coli have been the cause of serious food poisoning incidents in Australia.
As a consequence, the isolation of these pathogens by a laboratory is subject to mandatory notification requirements under the Regulations.
The notification of prescribed pathogens ensures WA Health can provide assistance to food businesses to effectively manage the risks posed by the presence of these pathogens in their foods or in their premises and ensure the safety of food available for sale in Western Australia.
The legal framework for notification
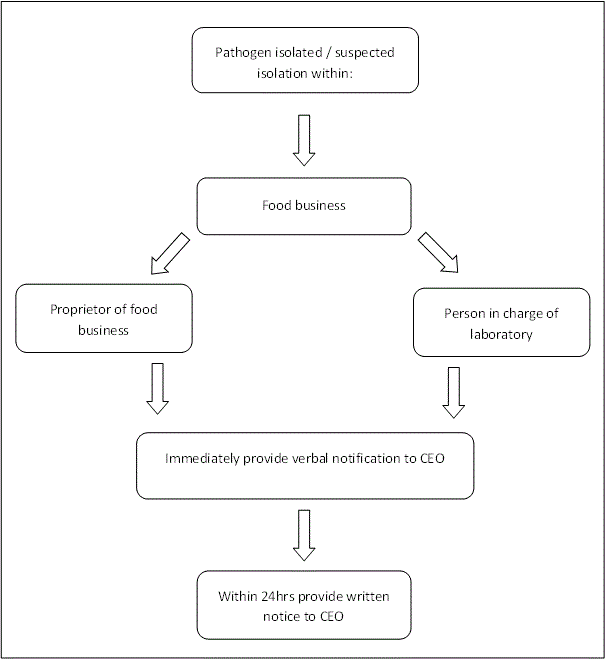
Part 4 of the Regulations requires that the proprietor of a food business must notify the Chief Executive Officer of the Department of Health (CEO) once they have been informed of the isolation or suspected isolation of a prescribed pathogen.
The person in charge of a laboratory is also responsible for notifying a prescribed pathogen in a WA food business to the CEO. It is important to note that the person in charge of the isolating laboratory is also responsible for notifying once the pathogen is isolated or is suspected to have been isolated.
It is important to note that this requirement extends to circumstances where a prescribed pathogen is suspected to have been isolated. Suspicion must be notified, even though actual isolation of Listeria monocytogenes has not been confirmed.
Scope of Part 4 of the Regulations
The notification requirements of Regulation 15 of Part 4 apply to all food businesses and analytical laboratories operating within WA. If however, analysis has been performed outside of WA, then the responsibility for notification is only required by the food business.
What will happen following notification?
An officer from the Food Unit or from local government may contact the food business to investigate the nature of the isolation and what measures, if any, have been undertaken by the food business to manage the potential risks.
Food businesses food safety responsibilities
Food businesses must ensure that they have procedures in place to effectively respond to a notification of the presence of a pathogen. This may include the activation of a food recall decision matrix, isolation of the batch sampled and undertaking of remedial corrective action.
The notification of the presence or suspected presence of a pathogen must always result in a review of food safety procedures and practices.
Procedure for notification
The flow-diagram below provides an overview of the procedure for notification. The information required for written notification is contained within the notification of suspected presence or isolation of pathogens form requirements along with a sample form (Word 384KB).
Last reviewed: 09-10-2020
Produced by
Environmental Health Directorate